Ionic bonding involves a transfer of an electron so one atom gains an electron while one atom loses an. We only notice that the covalent bond is the strongest chemical bond.

Covalent Bond Definition Types And Examples
Elements with large distances on the periodic table tend to form what bonds.
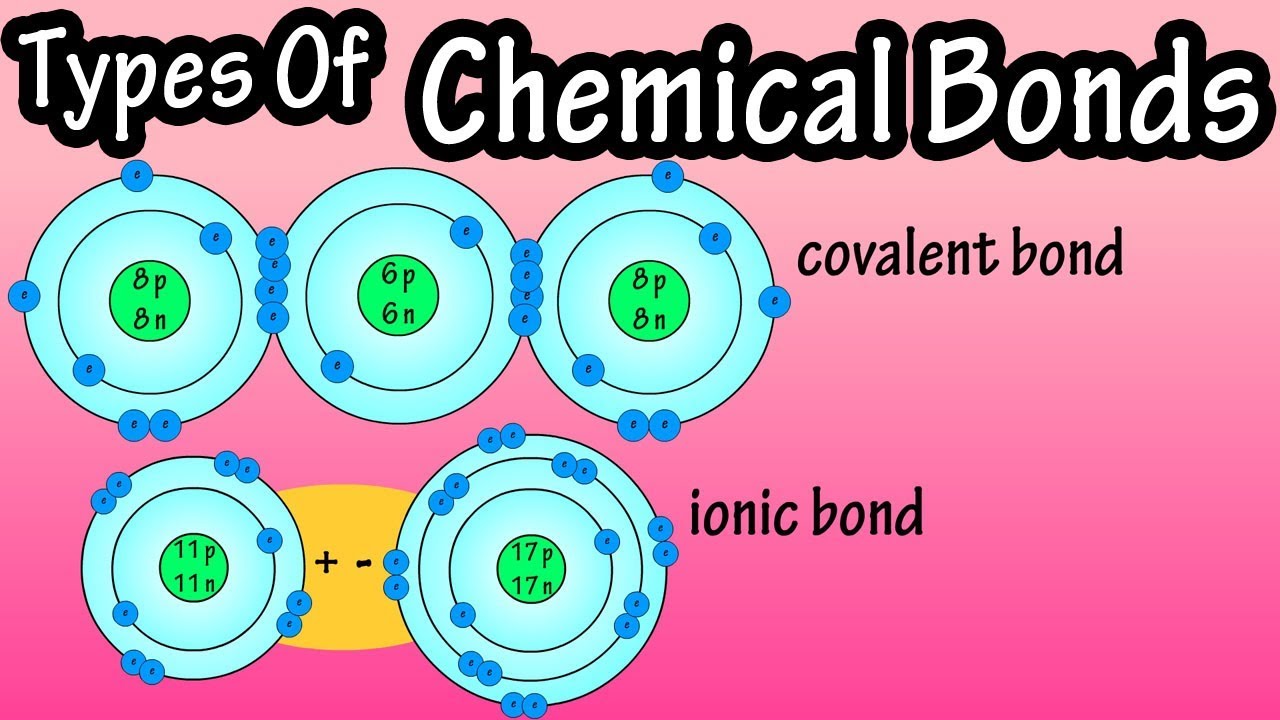
. Charged atoms or ions can form when atoms lose or gain electrons- remember that atoms will gain or lose electrons in order to have a full outer shell. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Chapter 4 Covalent Bonding and Simple Molecular Compounds.
These all involve electrons moving between atoms in various ways. Two atoms connected. Ionic Bonding Ionic bonds are formed when one atom or molecule transfers at least one electron to another atommolecule.
Hydrogen bonds refer specifically to the types of bonds formed with hydrogen atoms such as the water in sweat. Metallic bonds form between two metal atoms. Covalent bonds also known as molecular bonds.
Hydrogen attracts and bonds to neighboring negative charges. Atomic Structure Ions are atoms with negative or positive electric charges. Ionic and covalent bonds are strong interactions that require a larger energy input to break apart.
Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule. When an element donates an electron from its outer shell as in the sodium atom example above a positive ion is formed Figure 2. The atom that gains an electrons is called the electron acceptor.
Ionic covalent and hydrogen bonds. Intramolecular bonds An intramolecular bond is any bond that binds together the atoms making up a molecule or compound. Bonds formed between ions with opposite charges.
Ionic bonds are formed by the electrostatic attraction of atoms that have opposite charges. Atoms start off with the same number of positive protons and negative electrons. There are various types of chemical bonds including.
In a polar covalent bond the electrons are unequally shared by the atoms and spend more time. Types of chemical bonds There are three major types of chemical bonds. These 2 types of bonds are 1.
What are the three main types of bonds. What is an ionic bond. There are two basic types of covalent bonds.
The classical model identifies three main types of chemical bonds ionic covalent and metallic. Ionic covalent hydrogen bonds and van der Waals interactions. Usually there is some polarity polar covalent bond.
Chemical bonds can be classified into two types as Primary strong bonds-. Covalent bonds form between two non-metal atoms. Polar covalent bonds also known as polar bonds.
The covalent bond can be categorised into the following categories based on the number of shared electron pairs. Atoms bonded by sharing electrons. There are four types of bonds or interactions.
These types of chemical bonds include. The most common bond in organic molecules a covalent bond involves the sharing of electrons between two. These types of bonds in chemical bonding are formed from the loss gain or sharing of electrons between two atomsmolecules.
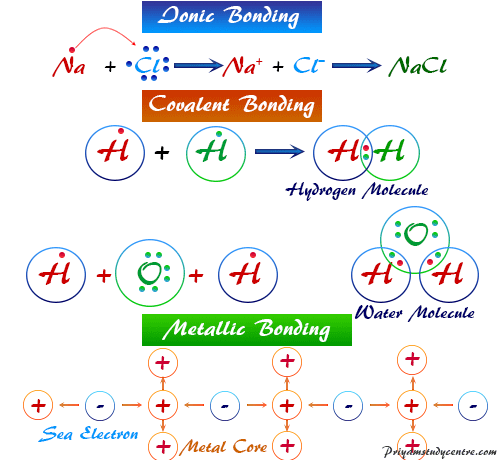
The three main types of bond are ionic covalent and metallic. In general there are 4 types of chemical bonds. An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms.
Ionic Covalent and Metallic. In this bond the electrons are. Polarized bonds form when two atoms share an electron but one of the elements has a stronger attraction to that shared electron.
There are mainly four types of chemical bonds 1IONIC BOND. The two main types of chemical bonds are ionic and covalent bonds. When metal and nonmetals bond what type of bond are they.
Chemical bonds allow all of the elements to combine in a variety of ways to create everything on Earth. Covalent chemical bond which in turn can be polar and non-polar Ionic bond Hydrogen bond Metallic bond As for the covalent chemical bond there is a separate article on our website and you can read more in the link. 4 Types of Chemical Bonds Ionic bond.
The only pure covalent bonds occur between identical atoms. 2COVALENT BOND 3CO-ORDINATE COVALENT BOND 4METALLIC BOND. The classical model identifies three main types of chemical bonds ionic covalent and metallic.
Another much weaker type is the hydrogen bond. Nonpolar covalent bonds form when the electronegativity values are very similar while polar covalent. What is an ion.
There are four types of chemical bonds essential for life to exist. Ionic bond is a chemical bond between atoms formed by the transfer of one or more electrons from one atom to the other. Hydrogen bonds often abbreviated to H-bonds.
If the electronegativity values of two atoms are similar. A brief description of the different types of chemical bonds can be found below. These are the bonds that are strong and difficult to break.
Ionic Bonds Covalent Bonds Dative Bonds Network Covalent Bonds Ionic Bonds One type of chemical bond is an ionic bond. This type of bond is also called the molecular bond. This way the opposite charges cancel each other out.
Nonpolar covalent bond polar covalent bond ionic bond Nonpolar covalent bond Formed by the equal or nearly equal sharing of electrons between two nonmetal metalloid atoms.

Chemical Bonds Definition Types And Examples
Chemical Bonding Definition Types Properties Examples

Types Of Chemical Bonds What Are Chemical Bonds Covalent Bonds And Ionic Bonds What Are Ions Youtube
0 Comments